Overcoming Challenges in Analytical Method Development for Colloidal Oatmeal OTC Monograph 产品s
The history and utility of colloidal oatmeal for dermatological applications
燕麦粥有几百年的历史, well-documented history of use as a skin protectant and anti-irritant, 缓解红, 有裂痕的, 和/或由各种原因引起的皮肤瘙痒, 包括刺激, 敏感性, 皮肤病和炎症. In 1945, 即食胶体燕麦片被引入市场, 1989年, colloidal oatmeal was proposed by the US 食物 and 药物 Administration (FDA) as a Category I ingredient, pending the standardization of its composition and concentration. Category I ingredients are generally recognized as safe and effective (GRASE) for the claimed therapeutic indication. 最终, the FDA approved colloidal oatmeal as a monograph ingredient to be used as a skin protectant in 2003.
When incorporated as an active ingredient in over the counter (OTC) drug products or personal care products marketed with claims of temporary protection and relief, 制定, product label and marketing claims must conform to the specifications outlined within the FDA OTC skin protectant monograph. If OTC monograph products comply with the requirements in section 505G of the Federal 食物, 药物, 和化妆品法案(FD&C法), including any relevant conditions detailed within an OTC monograph, 它们可能在没有批准药物申请的情况下上市.
胶体燕麦的作用机理及化学性质
The United States Pharmacopeia (独特销售主张) defines colloidal oatmeal as “the powder resulting from the grinding and further processing of whole oat grain meeting U.S. 1号或2号燕麦的标准(7 CFR 810).1001).“胶体燕麦片的化学成分很复杂, 主要由淀粉组成, 还有蛋白质, 脂质, 纤维, 和葡聚糖, 如图1所示.
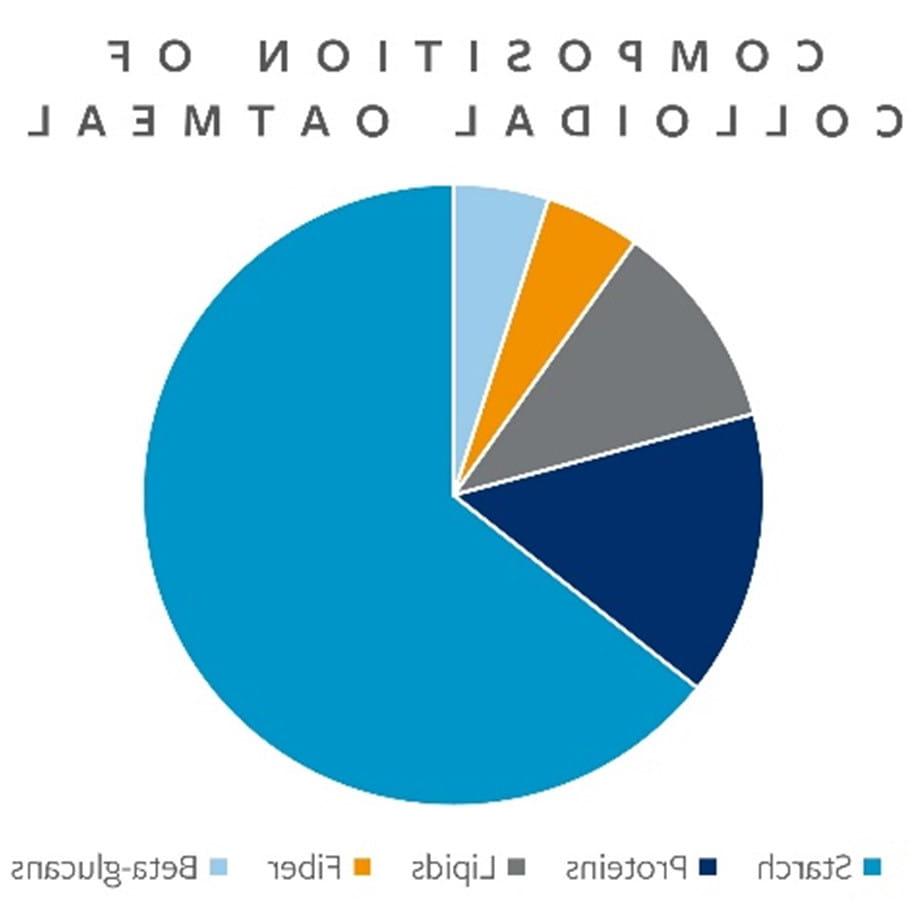
图1. 胶体燕麦片的组成
The diverse chemical makeup of colloidal oatmeal has been fundamental to its clinical utility and recognized role in managing symptoms associated with dermatological conditions such as atopic dermatitis and eczema. The anti-inflammatory and antioxidant properties of colloidal oatmeal are attributed to the multiple types of phenols present in the material, 还有一些抗氧化剂, 包括avenanthramides, 维生素E, 阿魏酸等. Avenanthramides, 燕麦中发现的关键多酚类抗氧化剂, are credited with having a significant impact on the inflammatory processes associated with atopic dermatitis and are also acknowledged with having antihistaminic activity. 此外, 胶体燕麦片吸水, 促进水分在皮肤中的保留, 因为它含有高浓度的淀粉和葡聚糖. 胶体燕麦片也有助于缓解瘙痒, 因为它的缓冲特性使皮肤的pH值正常化.
Overcoming challenges in colloidal oatmeal analytical method development
When developing robust and sensitive analytical methods to quantify the amount of colloidal oatmeal in a finished product, the complexity of both the polymorphism of colloidal oatmeal and the matrices of OTC products that contain colloidal oatmeal present challenges. Key to method development is the use of both placebo (product prior to the addition of colloidal oatmeal) and finished product. 由于护肤品往往是复杂的配方, mixing well to ensure a homogenous product is also essential for 精度 and 精度, 否则,结果将因采样而异. 此外, after methods have been validated per 我 guidelines for specificity, 精度, 线性, 范围, 精度, 再现性, LOD /定量限, 和鲁棒性, lots of finished product may be released per the validated method. It is important to note the exact lot of colloidal oatmeal used in finished batches of product must be used during lot release testing to ensure 精度 of results.
While many contract research organizations (CROs) and contract development and manufacturing organizations (CDMOs) have difficulty overcoming the challenges inherent to colloidal oatmeal method development, 验证和测试, im体育APP has a years-long proven track record of successfully developing and 验证 robust and sensitive HPLC-UV analytical methods to accurately quantify the amount of colloidal oatmeal present in OTC monograph products for lot and batch release.
Ensuring compliance to the FDA’s OTC monograph drug requirements
Provisions to modernize and reform the way OTC monograph products are regulated in the US took effect in March 2020 when the Coronavirus Aid, 救援, 经济安全法案(CARES Act)签署成为法律. OTC monographs apply to approximately 800 active ingredients for over 1,400种不同用途, 这导致了超过100的授权,000种药品. While some aspects of the OTC monograph drug regulation process stay the same, 变更包括行政命令流程, 对剂型进行微小改变的过程, 以及某些专卖药物的专卖期, 等.
im体育APP’s consultative teams of regulatory and scientific experts have the depth and breadth of experience and regulatory knowledge to ensure OTC monograph products comply with the FDA’s updated requirements for OTC monograph products. 经过多年的成功开发, 验证, and releasing OTC products with complex and challenging active ingredients and matrices, 包括胶体燕麦片, im体育APP is the partner of choice for leading OTC product developers and manufacturers. 今天就与专家取得联系 探索与im体育APP的合作关系.
找到相关的 资源
了解更多

分析方法开发和验证
im体育APP provides innovative analytical method development and validation solutions to regulatory guidelines for a wide 范围 of analytical technologies.

产品Deformulation
im体育APP’s expert chemists have successfully reverse engineered hundreds of formulations for a huge array of products and provided detailed information on the identity and quantity of ingredients.

Compendial测试
我, 独特销售主张, EP, 原料的BP和JP专著试验, 药物的物质/产品, 起始物料, 手机银行, 向量, 大量收获和控制细胞.
注册免费资源
Visit im体育APP's email subscription center to receive the latest industry news, 技术白皮书, 案例研究, 在线研讨会, 以及即将到来的活动.
