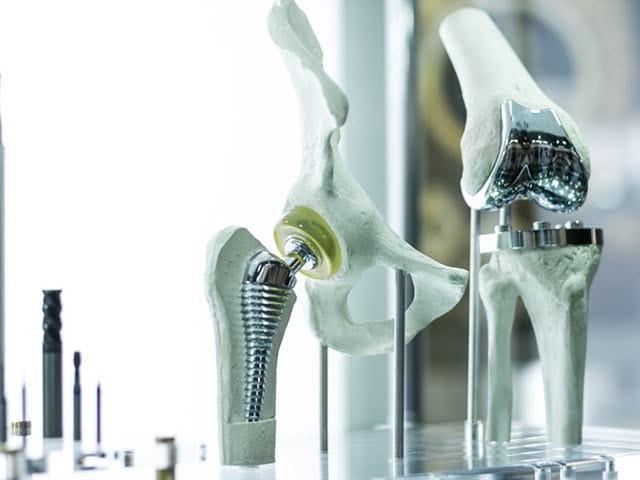
Element’s medical device product development services enable safe and quality products to reach the market faster. Our Engaged Experts have a clear understanding the regulatory requirements and challenges in the medical device industry to help you introduce new patented products and processes worldwide.
Product development process is a core process in any manufacturing activity. It ensures the final product meets the desired design and product specifications. It helps with product design, materials selection, evaluation of different fabrication approaches and development of a manufacturing process.
Medical device product development services
Element supports all phases of your medical device development: from initial concept development, through prototyping, optimization and small scale production.
We can design your product, fabricate molds and develop equipment required to manufacture your products. Any equipment we design, assemble, automate, troubleshoot and optimize can be transferred to your facility at the end of the project.
Our design engineers assist in navigating the transition between the prototyping and manufacturing of your device, whilst keeping safety, quality and compliance at the forefront.
We implement risk management and integrated quality management system (ISO 13485 and/or ISO 14971) assessments for all our medical device design and development services.
Bringing your device ideas to life
At Element, you’ll enjoy the benefit of a single source supplier for all of your testing needs. Our services include feasibility, R&D, verification and validation, product development, production quality control and product launching.
We offer a full suite of medical device testing including
For more information about our medical device product development and prototyping, contact us today.
Related Services

510(k) Testing
Successful FDA 510(k) submissions are critical to your market success. Incomplete or inaccurate submissions can lead to failure and may delay your product launch.

Medical Grade Polymer Testing
Polymers are some of the most commonly used materials in medical device manufacturing. Characterization of the relevant properties is key to ensure their quality and performance standards.

Test Protocol for Medical Devices
A protocol and plan will mitigate your risk, prevent confusion, set clear expectations, and preserve the necessary information for future reference and use.
Medical Adhesive Testing
Our medical adhesive testing ensures quality is maintained throughout the lifecycle of a product, mitigating risk to patients and your business.